| Peripatus Home Page | Updated: 01-Jun-2025 |
AbstractThis page presents a brief overview of the Trilobita, throughout their range from Cambrian to Permian.Keywords: Trilobita, trilobites, fossil history, systematics IntroductionTrilobites are extinct, exclusively marine arthropods, known fondly to everybody who has read much about fossils. In size, they ranged from a few millimetres to more than 60 cm, and are among the most common fossils – and the most distinctive index fossils – of their time. They appear suddenly in great abundance in the Cambrian, and evolved into a wonderful diversity, particularly during the Ordovician, before gradually dwindling away towards extinction some 300 million years later, in the Permian. An understanding of their history is central to any hypothesis of the development of the marine biosphere during the early Paleozoic. |
|
||||||||||
DescriptionTrilobites are characterised by a generally subelliptical, dorsal, chitinous exoskeleton divided longitudinally into three distinct lobes, a central elevated region and two less elevated lateral regions that run for most of the length of the body, hence their naming. (The name “trilobite” does not refer to the head, thorax and tail, which are found in many arthropods, but to the longitudinal division.) The following discussion notes only a few major morphological points; for a fuller and very accessible trilobite anatomy, see Rich et al. 1996, pp. 198-203. They have a distinct, relatively large head shield (cephalon) articulating axially with a thorax comprising articulated transverse segments, the hindmost of which are almost invariably fused to form a tail shield (pygidium). During growth, new segments were added in front of the pygidium and very young trilobites may look quite different to adults of the same species. Very little of the ventral surface was mineralised, and only those parts that were (the rostral plate and hypostome) are commonly preserved as fossils. The hypostome may have protected the trilobite’s mouth which lay above it and opened backwards. They appear to have protected their undersides primarily either by pressing their vulnerable ventral surface against the sediment or by rolling into a ball like an armadillo. The second method is believed to have been more effective where the cephalon and pygidium were about equal in size. Most trilobites used the upper parts of their front few pairs of legs to grasp food and push it forward into the mouth. In size, they ranged from a few millimetres (e.g. the agnostid, Peronopsis, fig. 1) to more than 60 cm (e.g. the Devonian form Terataspis, fig. 2). A new species of Isotelus measuring more than 70 cm was recovered from a Late Ordovician coastline exposed near Churchill, Manitoba, in 2001 (Rudkin et al. 2003). As far as is known, all trilobites were exclusively marine. However, they appear to have ranged throughout the full range of marine environments, both pelagic and benthic. |
(1) |
||||||||||
Phylogeny and EvolutionAffinitiesTrilobites are clearly arthropods. They exhibit a number of primitive arthropod features which, together with their very early appearance in the fossil record, have sometimes led to their being considered close to the main arthropod ancestral line. On the other hand, the trilobite eye is highly derived – there is nothing else like it in the arthropod (or any other) lineage – which belies this placement. |
|||||||||||
|
That they are extinct precludes molecular analysis, but early cladistic analyses based on morphological features (e.g. Wills et al. 1998) placed the trilobites, together with Naraoia and Molaria [→ sidebar] and adjacent to several other Burgess Shale forms, within the Arachnomorpha [→ sidebar]. Today, a consensus is beginning to emerge of a “trilobite clade” comprising the true trilobites and a number of hitherto poorly-understood forms, including the Naraoiidae, Liwiidae, Helmetiidae, Tegopeltidae, and Xandarellidae. (For a cladogram and fuller notes, refer to Sam Gon’s Trilobite Systematic Relationships page.) Molaria is probably more closely allied with the chelicerates than with the trilobite clade. Størmer (1944) proposed the name Trilobitomorpha to include the Trilobita and a miscellaneous collection of other Burgess Shale arthropods which he later (Størmer 1959) named the Trilobitoidea. The name Trilobitomorpha is not much used today, although a case could be made that it is available for naming the informal “trilobite clade” concept (e.g. of Cotton & Braddy 2000). Størmer (1959) did not formally designate a type for his “class” Trilobitoidea. However, the subclass Marrellomorpha (and so on down to the genus Marrella) is the first of several taxa listed immediately after. |
|
||||||||||
Fossil History |
|||||||||||
Fossil recordThe trilobite fossil record extends across some 300 million years. With the advent of mineralised hard parts, trilobites radiated rapidly and soon reached their peak clade diversity. They first appear in the Early Cambrian where the faunas are dominated by the orders Redlichiida and Agnostida. At times, trilobite lineages exhibit rapid evolutionary change, the basis for many zonal schemes both local and wide-ranging. Like other arthropods, the physical growth of trilobites was facilitated by moulting. With the hard exoskeleton being shed periodically and a larger new one being developed, individual trilobites were able to leave many fossils. This may in part explain why trilobites are among the most common fossils of their time. Origins“Trilobites are astoundingly diverse and common fossils in the Cambrian. They first appear as body fossils, represented by their calcite exoskeletons, at the base of Cambrian Series 2, approximately 521 million years ago (Zhang et al. 2017). The earliest trilobites exhibit marked geographic provincialism, with distinct clades representing the first records in Siberia, West Gondwana (Spain and Morocco), and Laurentia (western USA)” (Edgecombe 2020). The big problem with the earliest known trilobites, is that they are trilobites. That is to say, their earliest representatives – from the order Redlichiida and in particular the Fallotaspididae (Fig. 3) – are distinctly and emphatically trilobites, and they do not look like anything else. They provide few clues to which other arthropod groups may be their close relatives, or to their origins. Although it is true that one or two of the Ediacaran forms such as Spriggina (Fig. 4) superficially resemble early trilobites, to date the detailed case for such an ancestry is far from compelling. This problem is particularly galling in one respect: it has not escaped the notice of those well-known oxymorons, the creation science brigade. However, those of us with an interest in the origins of things are compensated with a fascinating puzzle. “The sudden appearance of trilobites in Cambrian Stage 2 and their pattern of endemicity have previously been taken to imply a prolonged unfossilised history. However, a Bayesian time tree for Cambrian trilobites, tip-dating using a morphological clock, predicts that the last common ancestor of Trilobita was Terreneuvian in age – Cambrian rather than Ediacaran (Paterson et al. 2019). Across a series of different clock models (e.g., allowing rates to vary within the four Series of the Cambrian but not between them, or varying between lineages but not systematically through time), diversification of trilobites was confined to the Cambrian. Trilobites thus require a shorter period of cryptic history than had been considered necessary to account for their early provincialism. This (inferred) wholly Cambrian record is congruent with trilobite-type trace fossils such as Rusophycus and Cruziana first appearing early in the Fortunian Stage, with no terminal Ediacaran record” (Edgecombe 2020). |
(3) Fig. 3: Fallotaspis typica (Hupe 1953) – One of the earliest occurring trilobites from the Lower Cambrian of Morocco. Overall length approximately 5 cm. After Moore 1959, fig. 133(5). Fig. 4: Spriggina floundersi Glaessner – An Ediacaran from the Vendian Pound Quartzite of Ediacara, South Australia. Overall length about 10 cm. Specimen from the Yale collection (YPM 63257). [Image courtesy of the Peabody Museum of Natural History, Yale University.] |
||||||||||
EvolutionThe earliest appearing trilobites belong to the suborder Olenelloidea (order Redlichiida, fig. 5) which is micropygous and, a little later, the secondarily blind Agnostida (fig. 1). Later in the Early Cambrian the Corynexochida and Ptychopariida appeared. The Late Cambrian was largely dominated by Ptychopariina (superfamilies Ellipsocephaloidea and Ptychoparioidea). Diversity remained relatively low, compared to the Ordovician, perhaps indicating a period of evolutionary stagnation (Clarkson 1993, p. 373). The mass extinction event at the close of the Cambrian introduced a major crisis in trilobite history; all Redlichiida, including the Olenelloidea, and most Late Cambrian stocks went extinct. A large positive excursion in carbon isotope values (SPICE event) and an imprecisely dated drop in Laurentian sea level (Sauk II – Sauk III event) are recorded from this time and may explain the extinctions. Predation by newly evolved cephalopods has also been suggested. |
(5) |
||||||||||
|
“Cluster analysis grouped families according to their numbers of component genera in each of the four Ordovician stratigraphic series ... showing diversity histories of all families [Fig. 6] indicate that the trilobites form two major clusters, termed faunas. The Ibex Fauna, named for the epoch during which it flourished, is characterized by Early Ordovician dominance followed by severe diversity reductions in the later Ordovician (conforming to a null hypothesis of cohort decay). The Whiterock Fauna, named for the epoch in which it radiated rapidly, displays a contrasting pattern of minimal Early Ordovician diversity, Middle Ordovician (Whiterock) radiation, and high Late Ordovician diversity. These alternate, disjunct patterns are pervasive and represent high-level macroevolutionary trends. Surviving Silurian families constitute a subgroup of the Whiterock Fauna, termed the Silurian Fauna. No member of the Ibex Fauna survived the end of the Ordovician. In contrast, nearly three-fourths of families (74%) of the Whiterock Fauna survived, and the Whiterock Fauna accounts for all post-Ordovician trilobites, with the exception of the unclustered harpetids” (Adrain et al. 1998).
Some short-lived Early Ordovician (Tremadocian) forms preceeded the principal Ordovician groups such as Seutelluina, Phacopida and Trinucleina. These principal forms were “all highly differentiated and diverse, most of them of crypogenic origin, and surviving for various periods thereafter. It is an interesting feature of trilobite evolution that after this great burst of constructional themes in the early Ordovician very few entirely new patterns of organisation arose; afterwards evolution in trilobites was largely a matter of [variations upon the Ordovician themes]” (Clarkson 1993, pp. 373-374). The Ordovician trilobites were more successful at exploiting new environments, notably reefs, but they too suffered a crisis, during the mass extinction at the end of the Ordovician. Some distinctive and previously successful forms such as the Trinucleoidea and Agnostoidea became extinct. Whiterock Fauna families, and in particular Silurian Fauna groups, can generally be traced only to the Early Ordovician, and in many cases they are entirely “cryptogenetic.” These clades certainly had Cambrian forebears, but the fact that they have avoided detection is a strong indication that novel morphologies were being developed very rapidly. Of the Silurian Fauna genera present during the Llandovery epoch, 78% also originated in that epoch, which demonstrates that extinctions were followed by a rapid post-extinction “rebound.” However, once this Llandovery rebound was completed, standing diversity returned to and was maintained at pre-extinction amounts. (After Adrain et al. 1998.) |
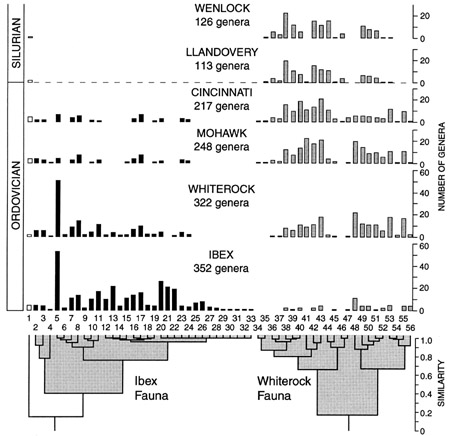 Fig. 6: Reproduction of figure 1 from Adrain et al. 1998, showing the two major clusters of Ordovician trilobite ‘fauna.’ |
||||||||||
|
Silurian and Devonian assemblages are superficially similar, dominated by Phacopida (Fig. 7), including the well-known Calymenina, and Lichiida (Fig. 8). However, a number of characteristic forms do not extend far into the Devonian and almost all the remainder were wiped out by a series of extinctions in the Middle and Late Devonian. Only the Proetida survived the Devonian, lasting through the Carboniferous and Permian periods up until the Permo-Triassic mass extinction event. The principal trends include the origin of new types of eyes, improvement of enrollment and articulating mechanisms, a change from micropygy to isopygy, development of extreme spinosity in certain groups (fig. 9), and a reduction of the rostral plate (that portion of the doublure enclosed by sutures in trilobites where the facial sutures extend across the anterior border of the cephalon). |
(7) Fig. 7: An Early Devonian phacopid, Reedops deckeri, from the Haragan Formation, Coal County, Oklahoma. [Image courtesy of Extinctions.com web site.] Fig. 8: A Silurian lichiid, Arctinurus boltoni (Bigsby), from New York State. Length approximately 12 cm. [Image courtesy of Extinctions.com.] (9) |
||||||||||
|
There was a world-wide trilobite extinction event in the Cambrian, in which large forms died out and were survived by much smaller genera. They were also one of the groups most affected by the end-Ordovician extinction, and most estimates record a loss of about half of familial or generic diversity at this event. “Because the Ibex Fauna was showing a general decline in diversity before the end of the Ordovician while the Whiterock Fauna was undergoing robust radiation, we infer that clade survival at the end-Ordovician extinction was related to clade size. ... [S]urvivors have larger genus diversity (mean, 8.3 genera) than those families that were extinguished (mean, 3.6 genera). The influence of clade size on survival contrasts with patterns of molluscan genus survival at the end-Cretaceous event, gastropod genus survival at the end-Permian event, and trilobite family survival at the end of the Cambrian, all of which were unrelated to clade size” (Adrain et al. 1998). Physical environmental changes were linked to the end-Ordovician extinction of some trilobite clades; the relatively sudden decimation of Whiterock Fauna families such as Cyclopygidae and Raphiophoridae is otherwise difficult to explain. End-Ordovician survival was markedly higher among Whiterock Fauna groups that initially diversified at low versus high latitudes. All eight Laurentian families survived the end-Ordovician extinction, compared with only three of the nine Gondwanan/Baltic groups. Groups that had a Laurentian early distribution account for the bulk of the Silurian Fauna. Most of the clades that became extinct were already in long-term decline, whereas most of the clades that survived suffered no sustained diversity reduction after the extinction. The last trilobites disappeared in the Permo-Triassic (Tatarian) mass extinction event. SystematicsToday there are nine generally recognised orders of trilobites: the Agnostida, Redlichiida, Corynexochida, Lichida, Phacopida, Ptychopariida, Harpetida, Asaphida and Proetida. |
| Order | Range | Synopsis | Suborder/Superfamily | Example |
| Agnostida | LC - Uq | Facial suturesmarginal; mostly lacking eyes; two (Agnostina) or three (Eodiscina)thoracic segments; isopygous. | Agnostina Agnostoidea Condylopygoidea Eodiscina Eodiscoidea | Agnostus Condylopyge Eodiscus |
| Redlichiida | LC - MC | Cephalonusually with elongate genal spines; sutures either fused or opisthoparian; eyes large; thoracic segments often spiny; micropygous. | Olenellina Olenelloidea Fallotaspidoidea Redlichiina Emuelloidea Redlichoidea Paradoxidoidea | Olenellus Fallotaspis Emuella Redlichia Paradoxides |
| Corynexochida | LC - MD | Opisthoparian; glabella expanded towards the front or parallel sided; commonly 7 to 8 thoracic segments; often macropygous. | Corynexochina Illaenina Leiostegiina | Olenoides Illaenus Chuangia |
| Lichida | MC - MD | Medium to large size; opisthoparian; glabella broad; often macropygous; pygidium characterised by three blade-shaped pleurae; carapace often tuberculated. | Lichoidea Odontopleuroidea Dameselloidea | Lichas Leonaspis Damesella |
| Phacopida | Lq - UD | Dominantly proparian though sometimes gonatoparian or opisthoparian; glabella variable; 8 to 19 thoracic segments; pygidium small in some early forms but typically medium to large. | Cheirurina Cheiruroidea Calymenina Calymenoidea Phacopina Acastoidea Dalmanitoidea Phacopoidea | Cheirurus Calymene Acaste Dalmanites Phacops |
| Ptychopariida | LC - UD | Dominantly opisthoparian, sometimes marginal or proparian; glabella usually tapering forward; more than three thoracic segments. | Ptychopariina Ellipsocephaloidea Ptychoparioidea Olenina | Ellipsocephalus Elrathia Olenus |
| Harpetida | UC - UD | Sutures marginal except on dorsal side at genal angles; glabella convex, narrowing forwards, with 1 to 3 pairs of furrows; cephalon with characteristic Harpid fringe, consisting of vaulted inner genal roll and outer bilaminar brim; thorax with 12 or frequently more segments; pygidium subtriangular, elongate to short. | Harpina Entomaspididae Harpetidae Harpididae | Entomaspis Harpes Harpides |
| Asaphida | UC - S | Opisthoparian; glabella with faint lateral furrows or smooth, commonly with glabellar tubercule; 6 to 9 thoracic segments; subisopygous. Sometimes considered (e.g. by Rich et al. 1996) to be a suborder of the Ptychopariida, the diagnostic feature is a ventral median suture. | Anomocaroidea Asaphoidea Dikelocephaloidea Remopleuridoidea Cyclopygoidea Trineucleoidea | Anomocaris Asaphus Ptychaspis Remopleurides Cyclopyge Trinucleus |
| Proetida | q - P | Genal spines present; opisthoparian; eyes large, holochroal; glabella large and bulbous; 8 to 10 thoracic segments; isopygous. | Proetoidea Aulacopleuroidea Bathyuroidea | Proetus Aulacopleura Bathyurus |
| Table1: Summary of the orders of trilobites. | ||||
|
A handful of families (some would treat the naraoiids this way) cannot be placed with certainty in any trilobite order. ReferencesAdrain, J.M.; Fortey, R.A.; Westrop, S.R. 1998: Post-Cambrian trilobite diversity and evolutionary faunas. Science 280: 1922-1925. Clarkson, E.N.K. 1993: Invertebrate Paleontology and Evolution (third edition). Chapman and Hall. Cotton, T.J.; Braddy, S.J. 2000: A "Big Hand" for the Chelicerates? Phylogeny of Arachnomorph Arthropods and the Origins of the Chelicerata. Young Systematists Forum. Edgecombe, G.D. 2020: Arthropod Origins: Integrating Paleontological and Molecular Evidence. Annual Review of Ecology, Evolution, and Systematics 51: 1-25. Paterson, J.R.; Edgecombe, G.D.; Lee, M.S.Y. 2019: Trilobite evolutionary rates constrain the duration of the Cambrian explosion. Proceedings of the National Academy of Sciences of the USA 116 (10): 4394-4399. Rich, P.V.; Rich, T.H.; Fenton, M.A.; Fenton, C.L. 1996: The Fossil Book. Dover. Rudkin, D.; Young, G.; Elias, R.; Dobrzanski, E. 2003: The world’s biggest trilobite ‐ Isotelus rex new species from the Upper Ordovician of northern Manitoba, Canada. Journal of Paleontology 77: 99-112. Størmer, L. 1944: On the relationships and phylogeny of fossil and recent Arachnomorpha, etc. Skr. Vid. Akad. Oslo, 1. Mat. Naturv. KI. 5. — 1959: Trilobitoidea. In Moore, R.C. (ed.) 1959: Treatise on Invertebrate Paleontology: Part O. Arthropoda 1. Geological Society of America: O23-O37. Whittington, H.B. 1981: Rare Arthropods from the Burgess Shale, Middle Cambrian, British Columbia. Philisophical Transactions of the Royal Society of London, Series B 292: 329-357. Wills, M.A.; Briggs, D.E.G.; Fortey, R.A. 1998: Evolutionary Correlates of Arthropod Tagmosis: Scrambled Legs. In Fortey, R.A.; Thomas, R.H. (ed.) 1997: Arthropod relationships. Systematics Association Special Volume Series 55 55. Zhang, X.; Ahlberg, P.; Babcock, L.E.; Choi, D.K.; Geyer, G.; et al. 2017: Challenges in defining the base of Cambrian Series 2 and Stage 3. Earth-Sci. Rev. 172: 124-139. |
| Peripatus Home Page |
Hits counted from 10 Sep 2018:
My Traffic Estimate